Neutron Source
Neutron Source
A neutron source for BNCT
In its present form, BNCT is based on epithermal neutron irradiation. Currently, the only facilities used as sources of neutrons for BNCT in Japan are the Kyoto University Research Reactor (KUR) Heavy Water Neutron Irradiation Facility and the cyclotron accelerator system at the Kyoto University Research Reactor Institute, shown in Photographs 1 and 2. Both nuclear reactors and accelerators have deceleration systems capable of converting the generated fast neutrons into epithermal neutrons. Figure 3 illustrates the energy distribution for the epithermal neutrons produced by these systems. The energy of epithermal neutrons is considered to range from 0.5 eV to 40 keV, taking into account the radiation weighting factor. To date, the KUR Joint-use Medical Treatment Group has verified the effectiveness of BNCT by offering the treatment to more than 500 patients using reactor neutrons and has also developed an accelerator neutron source for BNCT to facilitate wider use of the technique. In an effort to earn regulatory approval for the technique, the group began clinical trials on the treatment of recurrent glioma in October of 2012 and then on locally recurrent head and neck cancers in the spring of 2014 by using this accelerator– moderator system.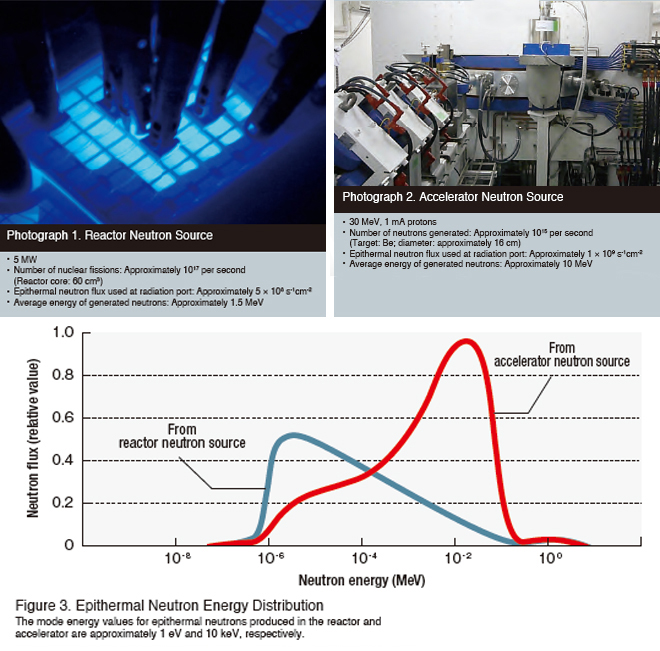
Irradiation field and internal dose distribution
Radiation at the BNCT epithermal neutron irradiation field consists of the following components: fast neutrons, epithermal neutrons, and thermal neutrons deriving from the produced neutron as well as gamma radiation given off by nuclear reactions in the deceleration system. Additionally, the primary types of radiation in the water phantom include incident radiation as well as recoiled protons and recoiled oxygen generated in the phantom and gamma radiation resulting primarily from 1H (n, γ) 2H reactions. Following administration of an intravenous boron agent, the internal dose includes not only the above radiation, but also alpha particles and 7Li derived from 10B as well as gamma radiation resulting from reactions between the elements that make up the body (for example nitrogen and sodium chloride in bodily fluids) and neutrons.
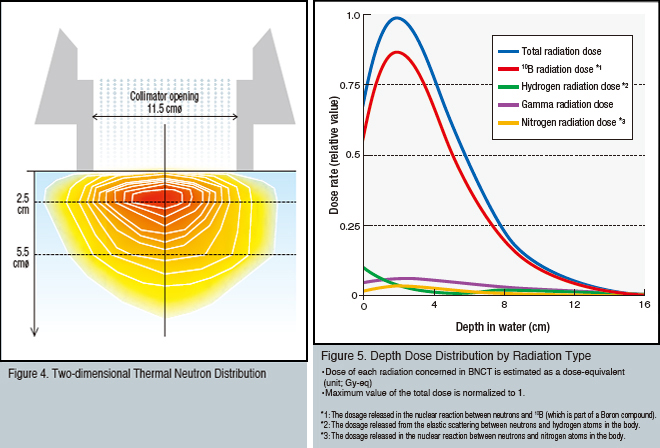
Figure 4 illustrates the distribution as epithermal neutrons are decelerated in the water phantom and thermalized. This data was observed during epithermal neutron mode irradiation at the KUR heavy-water irradiation facility. The peak depth of the thermal neutron flux is approximately 2.5 cm. This peak domain is about 5.5 cm in diameter, or approximately half the diameter of the collimator. Please note how broadening of the epithermal neutron direction of incidence and broadening due to scattering during the deceleration process in the phantom exceed similar increases seen in other radiation treatments. This characteristic determines the margin for BNCT planning target volume (PTV: the tumor volume for irradiation planning purposes, calculated based on three-dimensional image data of the tumor along with the extent of projected development, body movement, and irradiation field ambiguity).
Figure 5 illustrates the depth dose distribution of the 10B radiation dose, nitrogen radiation dose, hydrogen radiation dose, and gamma radiation dose inside a tumor with a 10B concentration of 30 ppm as well as the total distribution (total for all types of radiation) as calculated using the radiation dose and dose distribution plan. Under these conditions, 10B radiation accounts for approximately 85% of the total exposure. It is necessary to note that this proportion does not obtain uniformly throughout the irradiation field.
In order to assure the quality of BNCT, it is necessary to assess an extremely complex radiation composition as described above, to accurately evaluate the dose of each type, and to measure the benefit-versus-dose curve. Although it has not been implemented yet, real-time measurement of the 10B (n, α) 7Li and 14N (n, p) 14C reaction distributions in the body is the most important measurement issue that needs to be addressed.