Current BNCT in Japan
Current BNCT in Japan
Development of Compound Biological Effectiveness (CBE) factor and basic technologies in Japan
Along with the development of new boron compounds, important issues for BNCT include basic research to expand the application of existing compounds to common cancers and develop targeting techniques for using them effectively. Boron compounds exhibit a complex microscopic distribution, and the boron accumulation and dose vary with the types of cells that constitute normal tissue. Present research into the biological effects of radiation has not revealed how damage to individual types of cells contributes to overall tissue damage. Consequently, it is standard practice in BNCT to calculate a virtual dose based on the concentration of boron in the blood and the neutron fluence to the tissue, and then the X-ray equivalent dose is calculated by multiplying the virtual dose by a conversion coefficient. This coefficient indicates the effective relative biological effectiveness (RBE) of BPA or BSH, and it is known as the compound biological effectiveness (CBE) factor. Since the CBE factor changes depending on the kind of boron compound used and the type of tissue evaluated, it is determined by means of animal experiments. Japanese researchers have determined CBE factors of BPA for the skin and hepatocytes and of BSH for hepatocytes. This data has enabled BNCT to be used to treat malignant melanoma and liver cancer.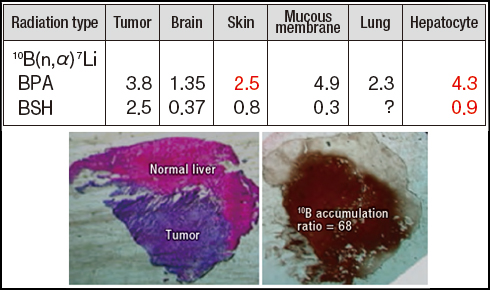
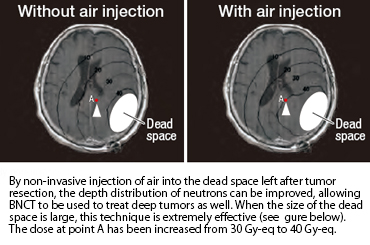 In order to facilitate the selective accumulation of boron compounds in tumors, KUR researchers devised the idea of applying the IVR procedure. It succeeded in selectively confining BSH to an experimental liver cancer using the IVR procedure, and a boron concentration ratio of about 70 was attained. In BNCT treatment of head and neck cancers, researchers are attempting to inject boron compounds into tumor arteries to facilitate super-selective drug delivery.
In order to facilitate the selective accumulation of boron compounds in tumors, KUR researchers devised the idea of applying the IVR procedure. It succeeded in selectively confining BSH to an experimental liver cancer using the IVR procedure, and a boron concentration ratio of about 70 was attained. In BNCT treatment of head and neck cancers, researchers are attempting to inject boron compounds into tumor arteries to facilitate super-selective drug delivery.
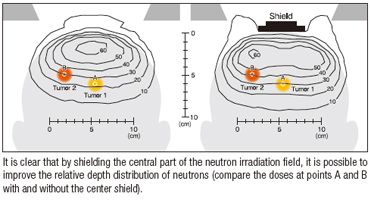 Unlike X-rays and heavy charged particle radiation, neutrons disperse like mist in the body upon irradiation. Consequently, their depth distribution can be improved by using techniques that seem counterintuitive from experience with X-rays and other kinds of radiation.
Unlike X-rays and heavy charged particle radiation, neutrons disperse like mist in the body upon irradiation. Consequently, their depth distribution can be improved by using techniques that seem counterintuitive from experience with X-rays and other kinds of radiation.
Our BNCT procedure (using a malignant brain tumor as an example)
The following figure illustrates the standard BNCT procedure for use in treating a malignant brain tumor, including prior medical examination, image inspection using CT and MRI, and confirmation of diagnosis by using FBPA PET.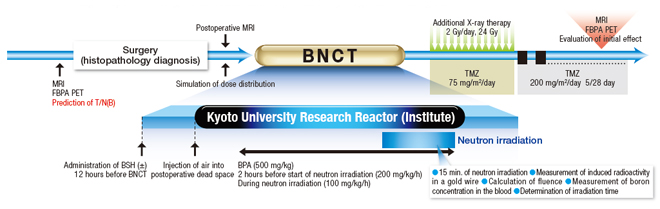
Improved thermal neutron beam facility
In addition to insufficient selective accumulation of boron compounds in tumors, the poor clinical results in the U.S. can be attributed to the low quality of the neutron beam that was used, that is, the mixing of an excessive level of gamma rays with a neutron beam. In Japan, an improved neutron beam port was used in clinical research. In particular, a heavy water facility capable of extracting thermal neutrons at a high level of purity was added to KUR. While this facility was not specialized for BNCT, it provided a high level of thermal neutron purity with a Cd ratio of 5,000 and a thermal neutron fluence rate of 6×109 n/cm2•s. A facility of the highest caliber, it was used in 1974 for the first BNCT at KUR.
From using thermal neutrons to using epithermal neutrons
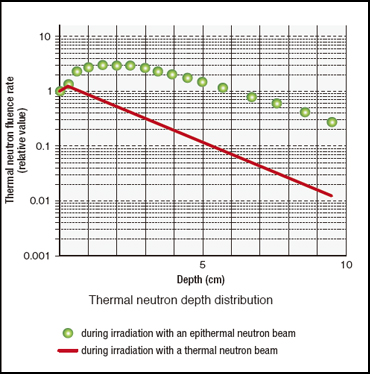 Research into BNCT became active again during the second half of the 1980s, especially basic research targeting malignant glioma, particularly glioblastoma. Epithermal neutrons lose their energy by degrees as they react with various atoms in the body and change into thermal neutrons. The depth distribution of thermal neutrons generated inside the body reaches its maximum value at a depth of 2 to 3 cm, about 3 times the value at the surface.
Research into BNCT became active again during the second half of the 1980s, especially basic research targeting malignant glioma, particularly glioblastoma. Epithermal neutrons lose their energy by degrees as they react with various atoms in the body and change into thermal neutrons. The depth distribution of thermal neutrons generated inside the body reaches its maximum value at a depth of 2 to 3 cm, about 3 times the value at the surface.
Furthermore, the attenuation of thermal neutrons in tissue is relatively gradual. Consequently, by using an epithermal neutron beam, no craniotomy is needed for neutron irradiation at the research reactor site when BNCT is applied to malignant brain tumors. In the autumn of 1994, BNCT was performed without a craniotomy at BNL’s research reactor in the U.S. using an epithermal neutron beam. In Japan, KUR’s attached heavy-water thermal neutron facility was modified so that it could also use epithermal neutrons from 1995 to 1996. However, the fixation on use of a thermal neutron beam was strong among researchers who studied BNCT, and large-scale use of epithermal neutron beams had to wait for the arrival of this century. In Japan, the modifications at KUR were followed by similar changes at facilities operated by the former Japan Atomic Energy Research Institute.
Appearance of FBPA PET
Since BNCT can be expected to yield clear effects with good accumulation of a boron compound, efficient treatment could be attained if such cancer patients could be selected by prior inspection.
Fortunately, BPA can be labeled using 18F, a positron-emitting radionuclide, and its accumulation in tumors confirmed by means of PET. Furthermore, the accumulation ratio in actual BNCT can be predicted by the ratio of 18F radioactivity in the tumor and that in normal tissue (blood). The world’s first BNCT procedure based on this prior inspection using FBPA PET was performed using KUR in February 1994, and it yielded the expected results.